December 13, 2017 TeleEMG, LLC % Barry Ashar President Makromed, Inc. 88 Stiles Road Salem, New Hampshire 03079 Re: K173441 Trad
RTsafe, Inc. ℅ Jennifer Palinchik President JALEX Medical, LLC. 30311 Clemens Rd. Suite #5D WESTLAKE OH 44145 Re: K180697 Tr

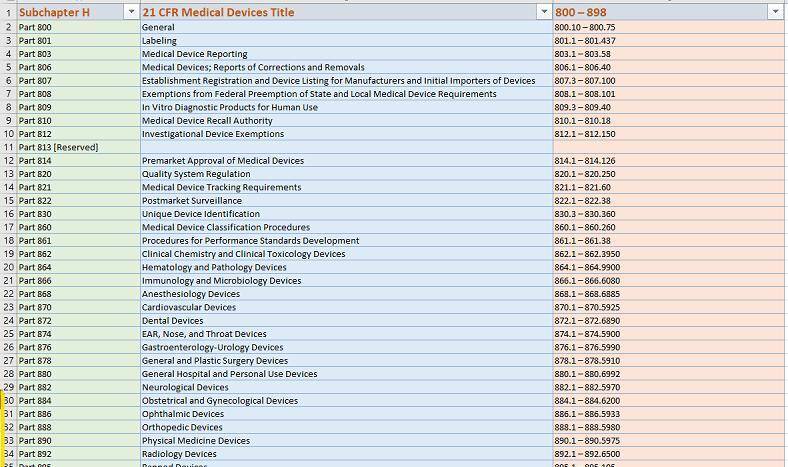
Code of Federal Regulations Title 21 Parts 800 to 1299 Food and Drugs: US Government Publishing Office, Office of the Federal Register: 9781683880820: Amazon.com: Books