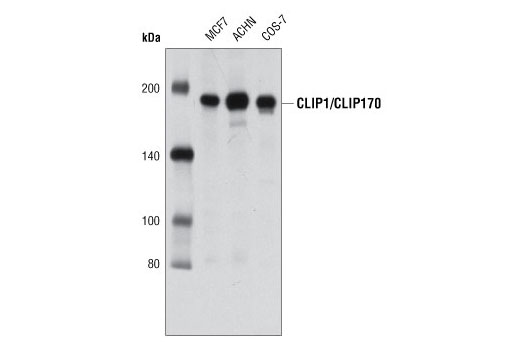
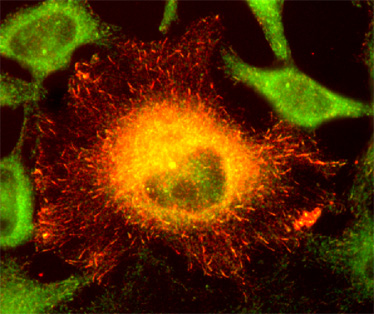
The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis

α-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons - ScienceDirect

The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE

Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | bioRxiv

AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation | Nature Cell Biology
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE

HIV‐1 capsids mimic a microtubule regulator to coordinate early stages of infection | The EMBO Journal

Ubiquitination of CLIP-170 family protein restrains polarized growth upon DNA replication stress | Nature Communications
Model for plus-end tracking activity of mammalian EB1 and CLIP-170. (A)... | Download Scientific Diagram
Model for plus-end tracking activity of mammalian EB1 and CLIP-170. (A)... | Download Scientific Diagram
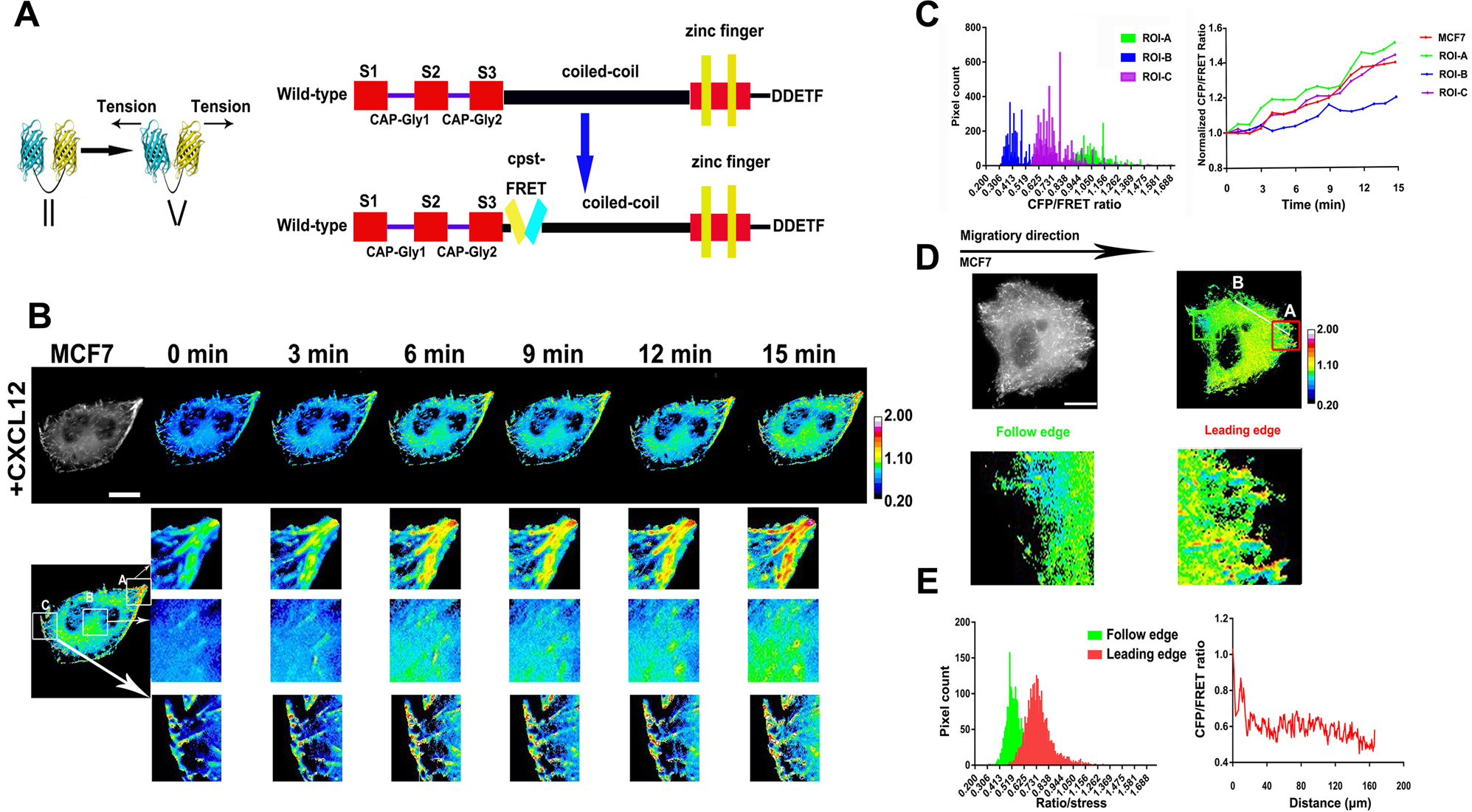
Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration | Cell Death & Disease
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE

LKB1 and AMP‐activated protein kinase: regulators of cell polarity - Nakano - 2012 - Genes to Cells - Wiley Online Library

The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis

Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition | PNAS
Potential mechanisms of microtubule plus-end tracking. (A) Motor-driven... | Download Scientific Diagram